Science | Dec 28, 2021
Properties of Solids, Liquids and Gases
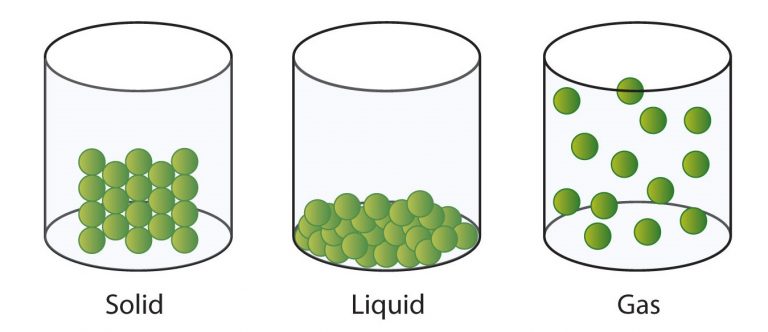
The three basic states of matter are:
- Solid
- Liquid
- Gas
Let’s talk about the properties of solid:
- Solids have fixed shape and volume with distinct boundaries (Figure 1).
- They have fixed volume.
- They have rigid nature which means their shape can not be changed as the particles are very tightly and closely packed.
- They can not be easily compressed as the gaps between the particles is very tiny so, it is very tough to compress them or we can say they have negligible compressibility.
- They have very high density.
- In context to vibration in solid, the particles vibrate back and forth within their fixed positions and can’t move freely.
- The kinetic energy of the solid particles is minimum and the intermolecular force of attraction is maximum in solid particles.
- Example of solids: solid ice, sugar, rock, wood, plastic, rubber, etc.
Properties of liquid:
- Liquids do not have definite shape as they can take up the shape of the container which means they can change its shape.
- They are fluids and they can be compressed easily and thus can flow like water.
- They have a very high density.
- The intermolecular forces between the particles of liquids are strong enough to have a definite volume.
- The particles are less tightly packed in case of liquids as compared to solids (Figure 2).
- The force of attraction between the particles of liquids is weaker than the solids.
- Examples of liquids: Water, mercury, milk, blood, etc.
Properties of gas:
- Particles in gas are far-away from each other (Figure 3).
- They don’t have definite shape and have no fixed volume.
- They can be compressed very easily and can take any shape.
- They have very low density.
- Gases particles have weak intermolecular forces between each other which means they can move in random motion or we can say freely.
- At room temperature, some elements exist as gas like Hydrogen, helium, fluorine, etc.
- Examples of gases: carbon dioxide, oxygen, helium, etc.
- Now if we speak about similarities between gases and liquids so, they both have:
- Definite shape.
- Can be cut through anywhere.
- Most importantly, can take up any shape means whatever is that the shape of the container gases and liquids can take up that shape.
- Gases and liquids also share another property which is Viscosity.
- Now if we discuss similarities between solids and liquids so, they both have:
- They have fixed volume.
- Both of them can’t be easily compressed.
Now, the question arises what are the physical properties of solids, liquids and gases:
- Solid, has a definite shape as well as has a definite volume. Coming to the liquid, they have a definite volume, but they will take up the shape of the container in whichever they are kept. And last talking about gases, they have neither a definite shape nor definite volume.














Post a Comment: